
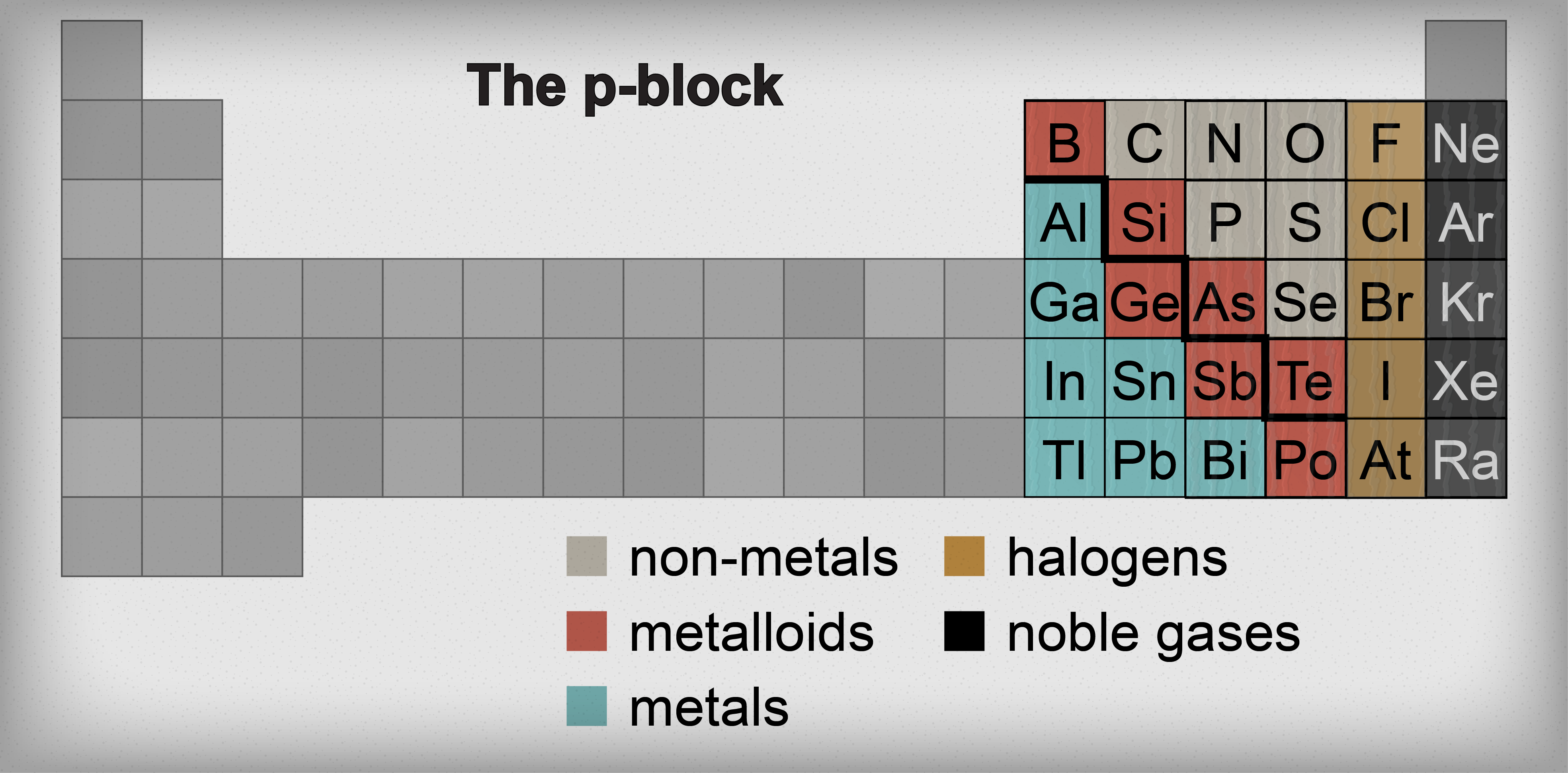
However, boron has one distinct difference in that its 2 s 22 p 1 outer electron structure gives it one less valence electron than it has valence orbitals. All three elements form covalent compounds. The metalloid boron exhibits many similarities to its neighbor carbon and its diagonal neighbor silicon. In this section, we will briefly discuss the chemical behavior of metalloids and deal with two of these elements-boron and silicon-in more detail. This intermediate behavior is in part due to their intermediate electronegativity values. For example, the pure metalloids form covalent crystals like the nonmetals, but like the metals, they generally do not form monatomic anions. Their chemical behavior falls between that of metals and nonmetals.

They are semiconductors because their electrons are more tightly bound to their nuclei than are those of metallic conductors. These elements look metallic however, they do not conduct electricity as well as metals so they are semiconductors. The metalloids are boron, silicon, germanium, arsenic, antimony, and tellurium.
Metalloids periodic table series#
Describe the preparation, properties, and compounds of boron and siliconĪ series of six elements called the metalloids separate the metals from the nonmetals in the periodic table.


 0 kommentar(er)
0 kommentar(er)
